China Approves Its First NMPA-Cleared Cardiac Electrophysiology Robotic System — TITIAN® EP Navigation and Ablation Robot
November 17, 2025

MEIO CardiNav Medical has received approval from China’s National Medical Products Administration (NMPA) for its TITIAN® Robotic Electrophysiology Navigation and Ablation System, making it the first EP ablation robot in China to complete multicenter clinical trials and obtain national regulatory clearance. The device was approved through the NMPA’s Innovation Pathway, underscoring its technical and clinical significance.
Transforming EP Ablation: From Radiation Exposure to Remote Precision Control
Atrial fibrillation ablation is a demanding, fluoroscopy-intensive procedure requiring precise catheter manipulation. Traditional manual workflows expose physicians to prolonged radiation and rely heavily on operator experience, making consistency difficult, especially with increasingly complex cases.
The fully self-developed TITIAN® System is the first EP interventional robot in China to complete multicenter clinical trials and obtain NMPA approval, and is supported by the national Innovation Green Channel program.
Superior operability:
TITIAN® is the first system in China to achieve dual-hand coordinated robotic control. Its dual robotic manipulators can jointly operate both the steerable sheath and the ablation catheter, fully reproducing the manual techniques of experienced EP physicians.
Enhanced safety:
A remote-operation workflow replaces bedside manual manipulation. During the procedure, the system provides real-time graphical visualization of the relationship between the sheath and cardiac anatomy, significantly improving safety margins.
Improved clinical effectiveness: Multicenter 12-month follow-up data show a 5.2% reduction in AF recurrence compared with manual procedures. Catheter contact stability increased by an order of magnitude, and pressure fluctuation was markedly reduced.
The system delivers millimeter-level, multi–degree-of-freedom precision, with integrated navigation for both the sheath and the ablation catheter. It supports rapid sterile turnover and catheter isolation workflows, enabling a complete “out-of–cath lab remote control” procedure.
System Capabilities and Compatibility
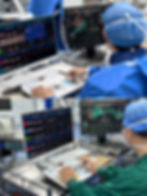
The TITIAN® platform includes a robotic execution unit, operator console, and workstation. It is compatible with mainstream pressure-sensing catheters, steerable sheaths, ICE catheters, and LAA occluder delivery systems, enabling seamless integration into existing EP workflows.
The robot supports both AF ablation and structural heart procedures such as LAA occlusion, reflecting a trend toward one-stop robotic EP and structural interventions.
A Milestone for Robotic Interventional Cardiology in China
The approval of TITIAN® signals a major advancement for robotic EP technology from China, introducing:
A shift from bedside manual manipulation to remote robotic precision
A pathway toward standardized, data-driven EP workflows
The emergence of a new robotic platform for arrhythmia and structural heart interventions
With global interest rising in robotic catheter navigation and radiation-free EP workflows, TITIAN® adds a significant new entrant to the rapidly evolving field of interventional cardiology robotics.
November 17, 2025, MedChina

